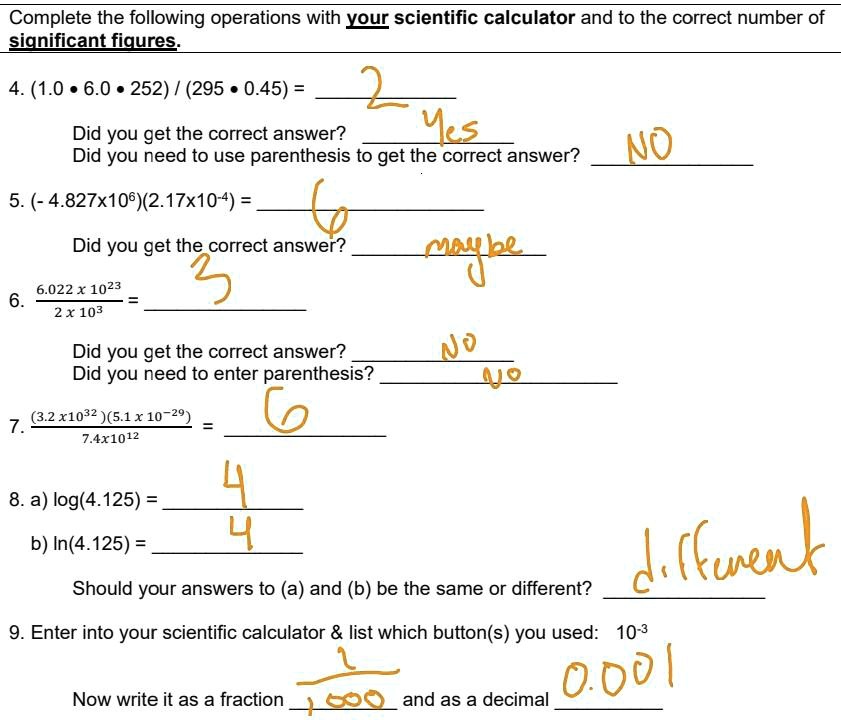
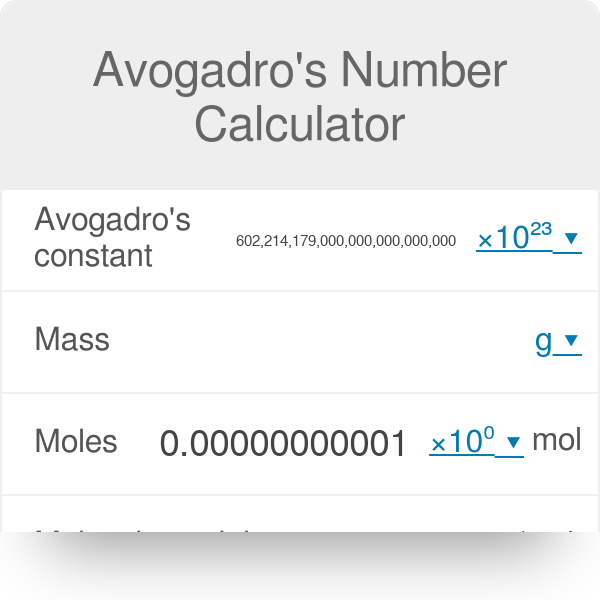
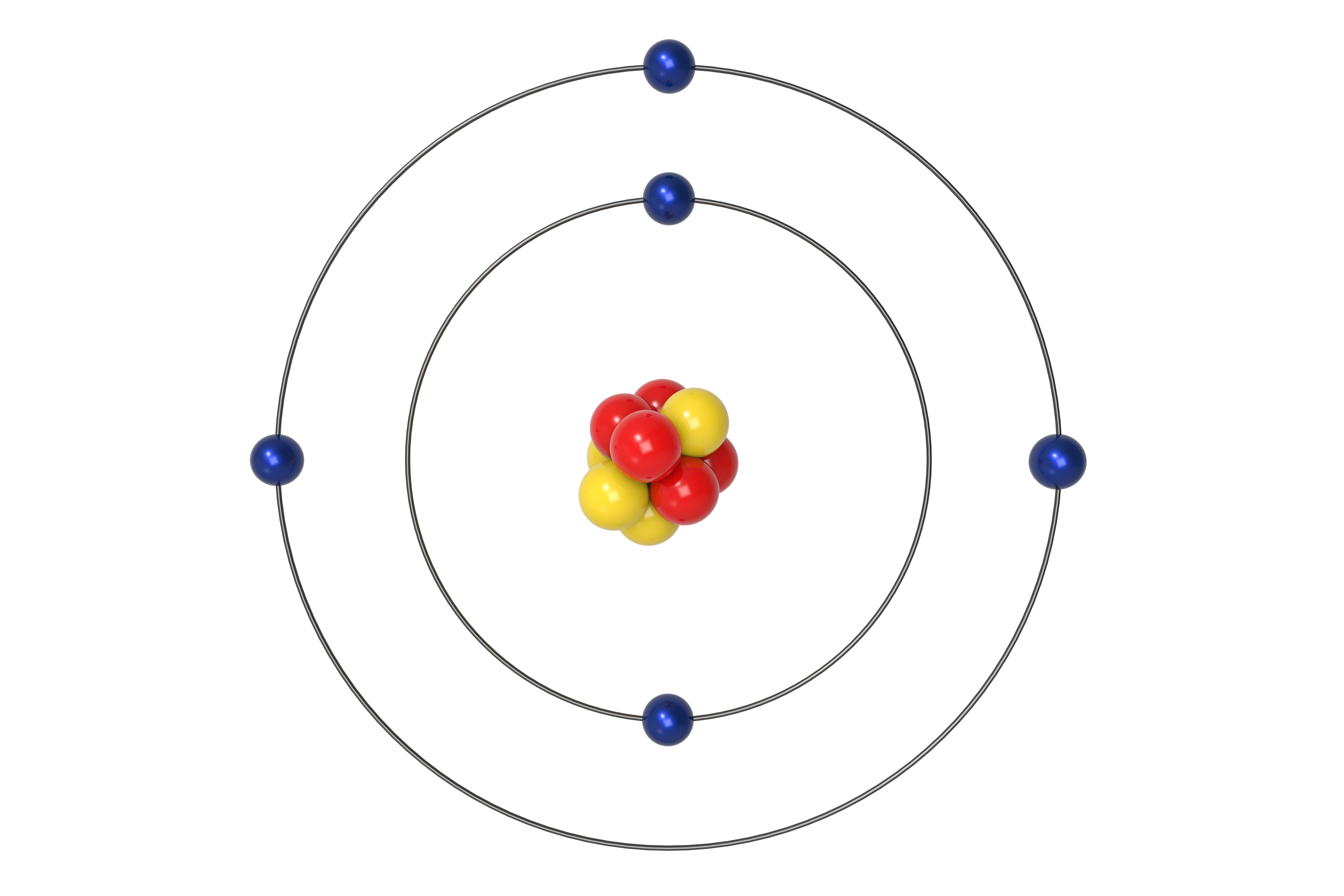
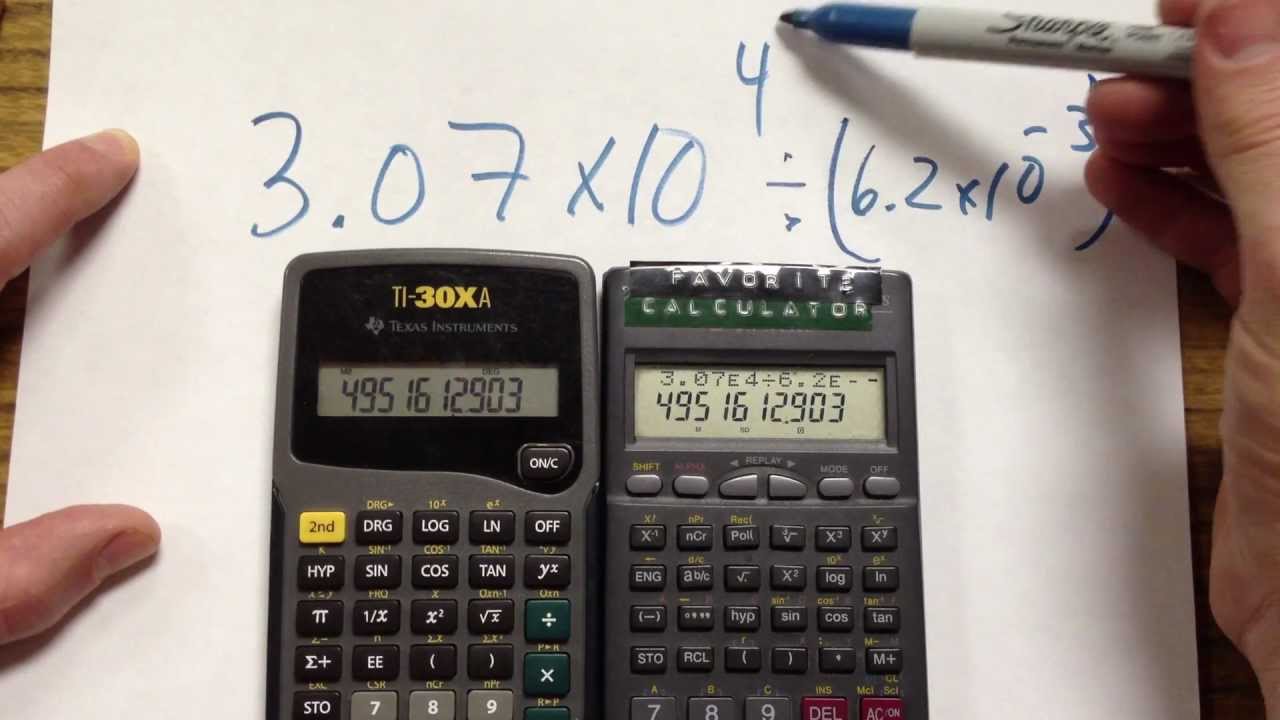How can atoms be counted? When we want to know how many atoms of a substance are in a sample of the substance that we can see, counting the atoms individually. -

How to Calculate Total Charge in Coulombs of an Arrangement of Protons and Electrons | Physics | Study.com
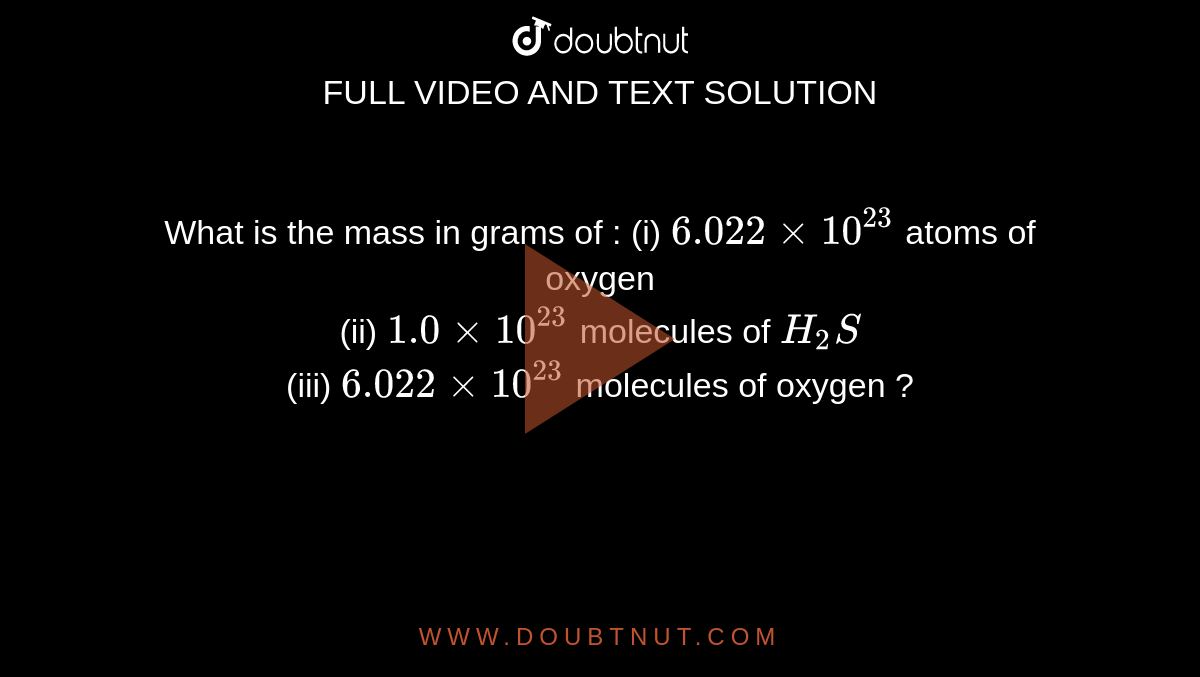
Determine the mass of the following : (i) 6.022 xx 10^(23) number of O(2) molecules (ii) 6.022 xx 10^(23) number of O atoms .

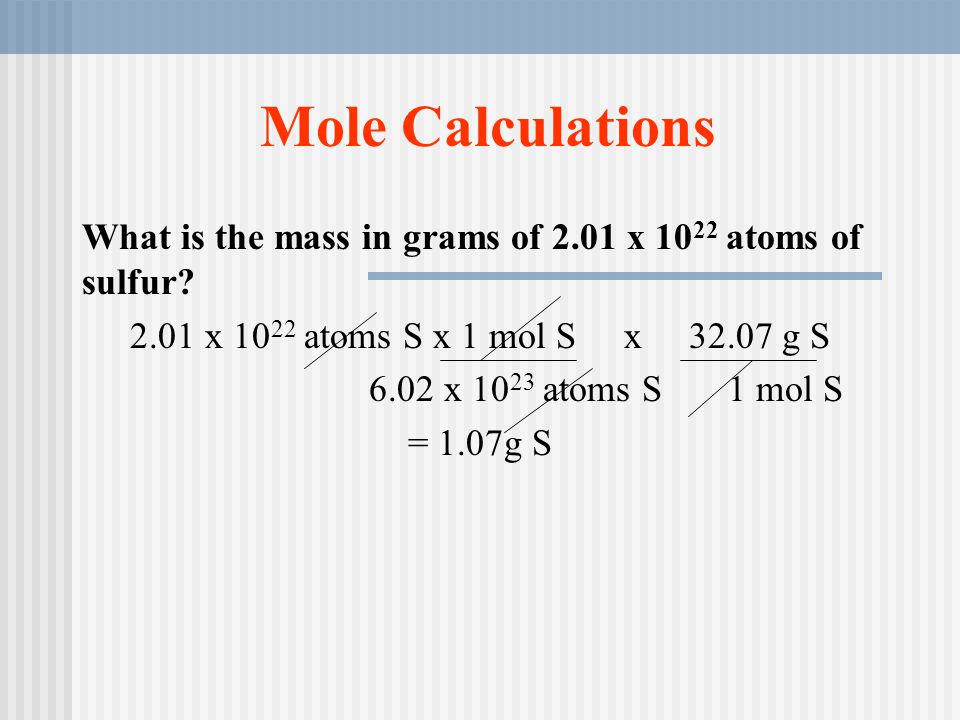
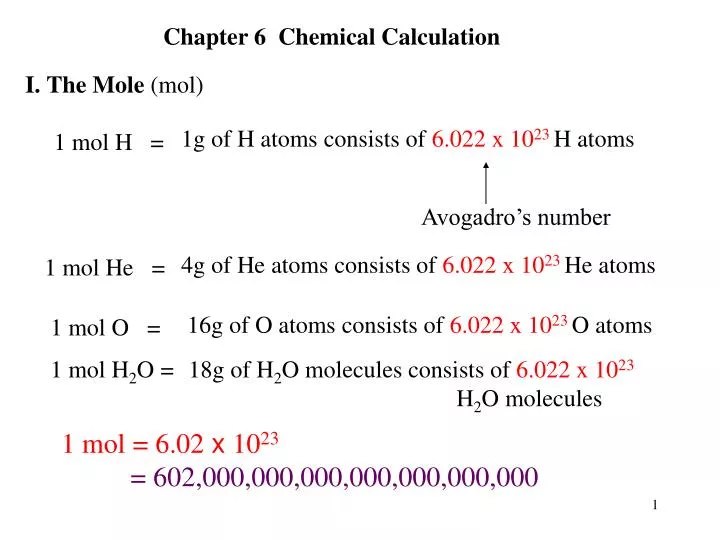
The Mole Concept. What is a mole? IIn chemistry, a mole is a counting unit. Abbreviated mol. 11 mol = 6.022x10 23 representative particles. Avogadro's. - ppt download

The Mole Concept. What is a mole? IIn chemistry, a mole is a counting unit. Abbreviated mol. 11 mol = 6.022x10 23 representative particles. Avogadro's. - ppt download

Calculate the mass of `3.011 xx 10^(24)` molecules of nitrogen gas `(N_(2))`. (Atomic mass : `N = 14 - YouTube

The Mole Notes. In every industry, there is a convenient way to measure a quantity of materials. For bakers, a baker's dozen = 13 For egg farmers, a dozen. - ppt download